We all see in the news that earlier today (Thursday, January 22) Microsoft had some massive internal crash that affected lots of people who had selected Microsoft (aka Outlook) to handle their email service.
I have seen this in two ways. A first way is that over the course of many hours, I was often unable to send an email to some person or another, and when I looked up the MX record (Wikipedia article) for that person’s domain, it would turn out to be “outlook.com”. Meaning that the person to whom I was trying (unsuccessfully) to send email had selected Microsoft (aka Outlook) to handle their email service.
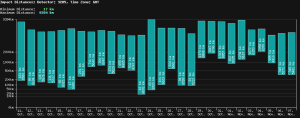
A second way is that our listserv server has been crippled by this outage. In a an average day our listserv server (see past blog postings here and here and how to donate) sends out a few tens of thousands of email messages to the members of our listservs. Each email gets sent once (in normal times) and that is it.
About one-third of the members of our listservs, it turns out, have selected Microsoft to be their service provider to handle their email.
Normally the chief way that this decision makes a problem for our listserv server is that Microsoft will wrongly make decisions to bounce our listserv emails as if they were spam (which of course they are not). As for this problem, what is desperately needed is for each member of our listservs (each member that uses Microsoft for email service) to contact Microsoft and tell them to stop doing that. (This includes, but is not limited to, telling Microsoft to whitelist our server’s IP address as described here.)
But a new and different way that this decision by many of our listserv members makes a problem for our listserv server arose today. Today, in a very intermittent and unpredictable way, Microsoft just sort of professed to be unable to receive any email at all (no matter who was trying to send the email), inviting the would-be sender to try again later. How much later, Microsoft did not say.
For our listserv server, this meant that our server would try again after a few minutes, and would get rebuffed by Microsoft, and would try again a few minutes later, and would get rebuffed by Microsoft again, and so on. This led to a massive queue of many thousands of outbound email messages that needed to be re-attempted over and over again. As for any particular listserv posting to some particular listserv member (who had selected Microsoft to handle that listserv member’s email service) this would eventually lead up to a reluctant decision by our server to abandon the efforts to send the email message.
Microsoft has not come out and said just what went wrong and has not come out and said they fixed it, whatever it was. But from reviews of our logs of outbound listserv emails, I get the impression that Microsoft may have fixed whatever it was that went wrong. Outbound listserv emails to listserv members who use Microsoft for their email seem to be back to their normal level of Microsoft randomly and wrongly discarding some percentage of our emails as if they were spam. Instead of bouncing all emails, Microsoft is back to bouncing only some percentage of them.
Again, if you are a member of one or more of our listservs, and if the company that you selected to handle your email is Microsoft, then please please tell Microsoft to whitelist our server’s IP address as described here.)



 CNN launched on June 1, 1980. Only now, 45 years later, has CNN become available “
CNN launched on June 1, 1980. Only now, 45 years later, has CNN become available “